|
Letter to Editor
Comparative analysis of different assays in assessing COVID antibody titer after ChAdOx1 nCoV-19 corona virus vaccine (recombinant) COVISHIELD vaccination
1 MD, MBA, Senior Consultant and Head, Department of Transfusion Medicine and Immunohematology, Sri Balaji Action Medical Institute, A-4, Paschim Vihar, New Delhi, India
2 MD, Senior Resident, Department of Transfusion Medicine and Immunohematology, Sri Balaji Action Medical Institute, A-4, Paschim Vihar, New Delhi, India
3 MBBS, MBA, Director, Sri Balaji Action Medical Institute, A-4, Paschim Vihar, New Delhi, India
4 MD, Senior Consultant and Head, Department of Microbiology, Sri Balaji Action Medical Institute, A-4, Paschim Vihar, New Delhi, India
5 MD, Senior Consultant and Head, Department of Biochemistry, Sri Balaji Action Medical Institute, A-4, Paschim Vihar, New Delhi, India
Address correspondence to:
Sadhana Mangwana
Department of Transfusion Medicine and Immunohematology, Sri Balaji Action Medical Institute, A-4, Paschim Vihar, New Delhi 110063,
India
Message to Corresponding Author
Article ID: 100012I03SM2021
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Mangwana S, Gohel D, Singhal D, Mutta J, Yadav D. Comparative analysis of different assays in assessing COVID antibody titer after ChAdOx1 nCoV-19 corona virus vaccine (recombinant) COVISHIELD vaccination. Edorium J Infect Dis 2021;6:100012I03SM2021.ABSTRACT
No Abstract
Keywords: Antibody titers, Covid vaccination, Neutralizing antibodies, Spike (s) protein
To the Editors
Currently, two vaccines against severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) have been approved for emergency use by the Indian Food and Drug Administration (FDA). COVISHIELD is a monovalent vaccine composed of a single recombinant, replication-deficient chimpanzee adenovirus (ChAdOx1) vector encoding the S glycoprotein of SARS-CoV-2. Following administration, the S glycoprotein of SARS-CoV-2 is expressed locally stimulating neutralizing antibody and cellular immune responses. Assessment of the safety and efficacy of vaccines against the SARS-CoV-2 in different populations is essential. In ongoing clinical trials, the COVISHIELD vaccine has been shown to prevent COVID-19 disease following two doses given between 4 and 12 weeks apart. The duration of protection against COVID-19 disease is currently unknown. Serologic tests for SARS-CoV-2 are antibody-based assays that measure an individual’s humoral immune response to SARS-CoV-2. This study was undertaken for estimation of COVID-19 antibodies in post-vaccinated volunteers and to compare the results of COVID antibody titer assays using three different platforms.
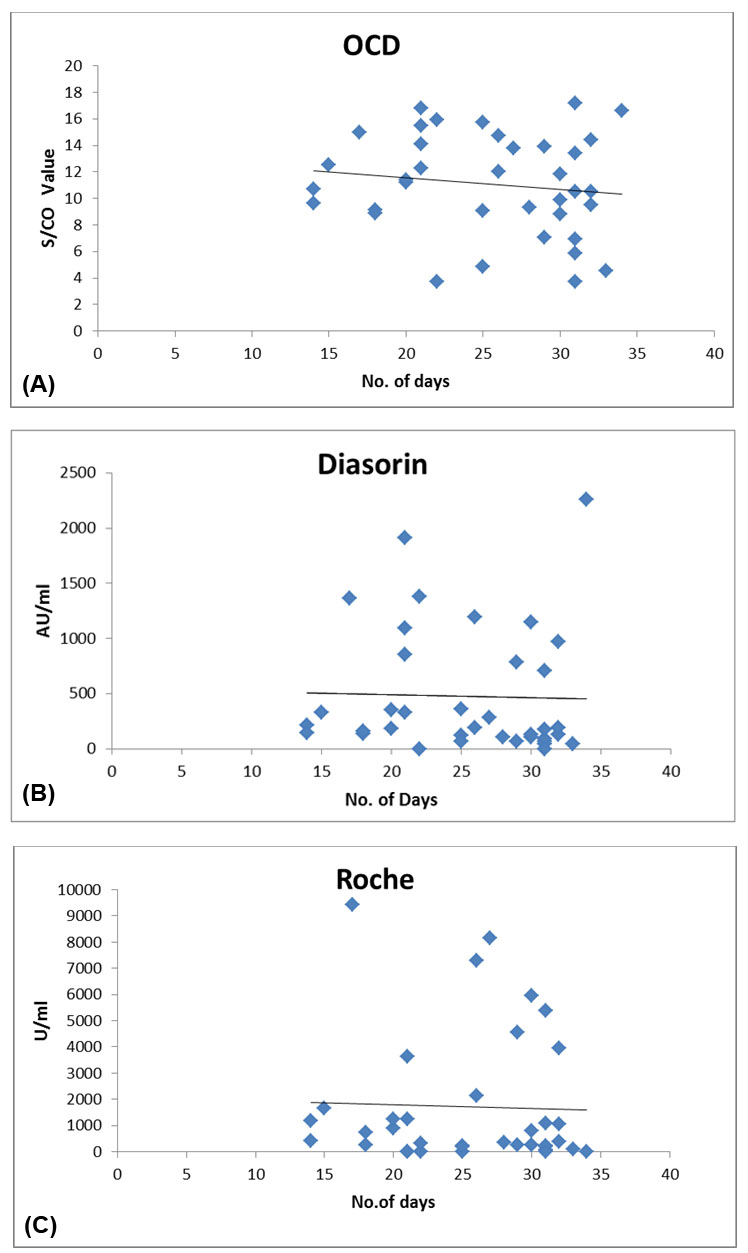
In this study, we determined COVID antibody titers after two doses of ChAdOx1 nCoV-19 corona virus vaccine (recombinant) COVISHIELD vaccination given at four weeks interval in 37 healthcare volunteers of which seven participants had confirmed SARS-CoV-2 infection before vaccination. Serum samples from 37 volunteers were collected between 15 and 34 days after second dose and tested by three, Indian FDA approved, in vitro assays; after approval by Institutional Research Board, which detected neutralizing IgG antibodies, Anti-SARS-CoV-2 against subunit S1 alone or both S1 and S2 subunits or S protein as a whole by semi-quantitative or quantitative methods having a sensitivity and specificity of ≥99.5% (Manufacturers—Ortho Clinical Diagnostics’ (OCD) Anti-SARS-CoV-2 IgG, Diasorin’s Liaison SARS-CoV-2 S1/S2 IgG and Roche’s Elecsys Anti-SARS CoV-2S Test Kits, respectively). Neutralizing antibody assays are correlated by vesicular stomatitis virus (VSV)-based S neutralizing capacity or plaque reduction neutralization test (PRNT). Results were analyzed and compared for different parameters. Assay ranges of respective kits of OCD, Diasorin, and Roche were “Not given,” “up to 400 AU/mL,” and “0.40–250 U/mL” with cutoff values as 1, 15 AU/mL, and 0.8 U/mL, respectively.
There were total 20 male and 17 female participants with age ranged from 26 to 77 years. It was observed that high level of antibody values were found in participants with 14–21 days post vaccination. Based on guidelines issued by US FDA dated March 9, 2021 and study at Mayo Clinic by Joyner et al. [1],[2], antibody values were categorized as low and high titers as S/CO(OCD Kit) less than 9.5 and more than 9.5, respectively. 24 participants showed high titer values (S/CO more than 9.5), with 54% participants (n = 13) as females in reproductive age group and 46% participants (n=11) were males. These participants showed values ranging from 125 to 2260 AU/mL by Liaison SARS-CoV-2 S1/S2 IgG (Diasorin) and 426.2–9441 U/mL by Elecsys Anti-SARS-CoV-2S (Roche). Seven participants with previous confirmed SARS-CoV-2 infection before vaccination showed S/CO values ranging from 11.8 to 17.2. These participants showed high values by Liaison and Roche Kits as well, with values ranging from 281–2260 AU/mL (Liaison, Diasorin) and 5383–9441 U/mL (Elecsys, Roche) (Figure 1). Two volunteers who have previously suffered from SARS-CoV-2 infection showed immeasurable high values by Elecsys Kit (Roche) of which one volunteer showed immeasurable values by both Liaison (Diasorin) and Elecsys (Roche) kits. This 77-year-old male participant has received convalescent plasma during his COVID illness.
Differences in performance of antibody assays can be explained due to the reason that kits are based on different principles of detection of antibodies to specific component of spike (s) protein, whether S1 subunit, S1 and S2 subunits, or S protein as a whole. Since antibodies to S1 protein or receptor binding domain (RBD) block the virus to ACE2 receptor, they are called neutralizing antibodies. Their generation is an important mechanism of humoral immunity against viral infection and is main goal of vaccines. The direct assessment of protective immunity in vivo is difficult so in vitro virus neutralization and associated surrogate neutralization assays are being developed to provide the best estimate of neutralizing potential.
ChADOx1 recombinant adenovirus vaccine is developed using codon optimized S glycoprotein and synthesized with the tissue plasminogen activator (tPA) leader sequence at 5 end. The adenovirus vector genome is constructed in the bacterial artificial chromosome by inserting the SARS-CoV-2S gene into the E1 locus of ChAdOx1 adenovirus genome. The virus was then allowed to reproduce in the T-Rex 293 HEK (Human Embryonic Kidney 293) cell lines and purified by the CsC1 gradient ultracentrifugation. The absence of any sub-genomic RNA (sgRNA) in the intra-muscularly vaccinated animals from the pre-clinical trials is indicative of the escalated immunity against the virus [3].
Each assay has its limitation and each laboratory should determine its right fit and acceptable performance to decide about kit. OCD’s assay, although detecting anti-SAR-CoV-2 IgG against S1 subunits spike protein, is a qualitative or semi-quantitative detection of IgG antibodies to SARS-CoV-2. Diasorin’s Liaison SARS-CoV-2 S1/S2 assay is quantitative assay determining anti-S1 and anti-S2 IgG antibodies and immune status of the person, having concordance with PRNT. Roche’s Elecsys anti-SARS-CoV-2S is a quantitative assay to determine antibodies to SARS-CoV-2 spike (s) protein RBD.
Without proven correlation of protection for SARS-CoV-2 vaccines in humans by surrogate markers, protective immunity cannot be measured effectively for recommendation of effective immunization program. Duration of antibody responses and other potential factors of protective immunity need to be investigated further with long-term, well-established, surrogate marker assays.
REFERENCES
1.
Emergency Use Authorization Declaration. 2021. [Available at: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#coviddrugs]

2.
Joyner MJ, Senefeld JW, Klassen SA, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: Initial three-month experience. medRxiv 2020;1–31. [CrossRef]

3.
Kaur SP, Gupta V. COVID-19 vaccine: A comprehensive status report. Virus Research 2020;288:198114. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Acknowledgments
We hereby thank Ortho Clinical Diagnostics, India; Diasorin S.p.A. and Roche Diagnostics GmbH for supporting this study.
Author ContributionsSadhana Mangwana - Conception of the work, Design of the work, Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dolly Gohel - Acquisition of data, Analysis of data, Drafting the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Deepika Singhal - Conception of the work, Design of the work, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Jyoti Mutta - Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Dinesh Yadav - Acquisition of data, Revising the work critically for important intellectual content, Final approval of the version to be published, Agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementThe requirement for written informed consent was waived, as participants volunteered themselves and self-enrolled after they had reviewed a study information letter and were given the opportunity to ask questions.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2021 Sadhana Mangwana et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.