|
Case Report
Direct inoculation of cutaneous blastomycosis from dog to human
1 MBBS, Amrita Institute of Medical Sciences, Cochin, India
2 MD, Geisinger Medical center, Danville, Pennsylvania, United States
3 MBBS, Kalinga Institute of Medical Sciences, Bhubaneswar, Odisha, India
4 MBBS, Government Medical College Alappuzha, Alappuzha, India
Address correspondence to:
Aparna Baburaj
472 Avenue D, Danville, Pennsylvania 17821,
USA
Message to Corresponding Author
Article ID: 100010I03AB2019
Access full text article on other devices

Access PDF of article on other devices

How to cite this article
Baburaj A, Sudhakaran A, Singh D, Chand S. Direct inoculation of cutaneous blastomycosis from dog to human. Edorium J Infect Dis 2019;5:100010I03AB2019.ABSTRACT
This is the case of a 46-year-old diabetic male presenting with non-healing ulcers on his face and lower extremity. He presented with two active crusted non-painful, mildly erythematous cutaneous lesions on his left foot and left chin that was present for two weeks. He had a prodrome of flu-like illness before noticing the lesions. Similar lesions were seen in the skin of his canine two weeks ago before it was noticed in the patient. His routine lab work including complete blood count and comprehensive metabolic panel were within normal limits. A chest X-ray showed normal lungs without any nodularity. A punch biopsy was done that showed cutaneous blastomycosis. He was started on Itraconazole with significant improvement in his cutaneous ulcer.
Keywords: Canine, Cutaneous, Cutaneous blastomycosis, Direct inoculation, Non-traumatic
INTRODUCTION
Blastomycosis is usually contracted by inhalation of airborne conidia and primarily affects the lungs manifesting as acute pneumonia or chronic pulmonary infection [1]. They can invade other organ systems via lymphohematogenous route and present as disseminated blastomycosis. Extrapulmonary conditions occur approximately in 25–30% of patients due to hematogenous dissemination from the lungs to other organ systems [2]. Hematogenous spread from the pulmonary system occurs most commonly to the skin [3]. Although rare, primary cutaneous blastomycosis can also develop with inoculation through direct trauma to the skin. Often, the cases of cutaneous blastomycosis are mistaken for malignant neoplasms and hence may not be discovered until further testing and biopsy [2].
We present the case of a patient who had cutaneous blastomycosis from direct inoculation from his dog without any obvious trauma. Even though direct inoculation cutaneous blastomycosis has been reported, this is a rare case of inoculation blastomycosis where the source of infection was from canine, and the spread occurred without any obvious trauma. We suggest that physicians should have a high index of suspicion for cutaneous blastomycosis in patients presenting with nonhealing ulcers from endemic regions where blastomycosis has a high prevalence. Physical contact with animals should be enquired, and if infection suspected, they should be treated as untreated animals could remain a nidus of infection.
CASE REPORT
A 46-year-old diabetic male from Oneida, Kentucky with past medical history significant for insulindependent type 2 Diabetes mellitus and essential hypertension presented with a chief complaint of right foot pain. He reported an active crusted non-painful, mildly erythematous cutaneous lesions located on left foot for two weeks that started with a prodrome of flu-like illness. It started as a small bump on his leg. He later noticed a similar lesion on his face. The lesions gradually increased in size. He denied any cough, shortness of breath or any bone pain. There was no significant discharge from the lesion
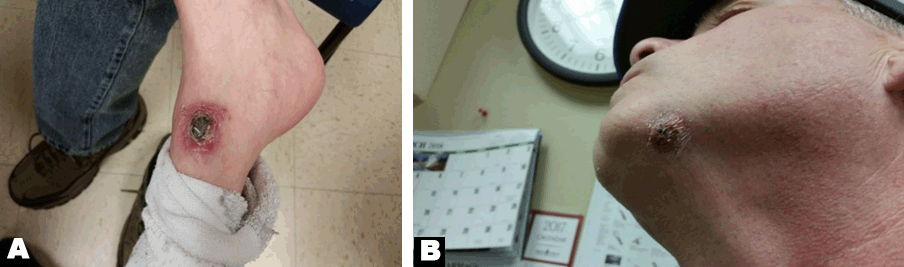
He was evaluated at his primary care physician’s office and was started on a course of antibiotic therapy without any improvement in his lesions. He had an incision and drainage of the lesion, and the specimen was sent for culture and sensitivity which came back negative for any bacterial growth. Due to the crusted and erythematous appearance of the lesion a biopsy was ordered with suspicion of squamous cell cancer. He was admitted to the hospital with worsening lesions and had a punch biopsy of the foot lesion. His physical examination showed lesions as shown in (Figure 1A) and (Figure 1B). At the time of admission, his vital signs were normal. Respiratory examination and cardiovascular examination were within normal limits.
Further history revealed that the patient had a hunting dog with similar lesions noticed two weeks prior. The patient had never gone for hunting and never been exposed to places where blastomycosis is prevalent but the dog he recently adopted from his friend have been used for hunting and has been to places where blastomycosis is widespread. He also did not have a history of travel outside of the united states.
His routine lab work including complete blood count and comprehensive metabolic panel were within normal limits. A chest X-ray showed normal lungs without any nodularity or hilar enlargement. X-ray of the foot was negative for any fractures or signs of osteomyelitis. He had a punch biopsy of his foot lesion that showed cutaneous blastomycosis with reactive pseudoepitheliomatous hyperplasia and abscess formation. Pas staining demonstrated rare fungal organisms with broad-based budding, morphologically compatible with blastomycosis.
After the biopsy results, the patient was started on Itraconazole. Two weeks of starting itraconazole therapy, his lesions showed significant improvement. A follow up after 6 weeks showed complete resolution of his lesions.
DISCUSSION
Blastomycosis is a fungal infection caused by a dimorphic fungus, Blastomycosis dermatitidis. The disease is endemic to areas of the United States and Canada including the great lakes region and the Mississippi and Ohio river valleys [4]. Exposure to inhaled conidia results in primary pulmonary infections and later spread to different parts of the body. This happens when contaminated soil containing fungal spores are disturbed by activities such as excavation, construction or wood clearing. Most of the exposure occurs when humans or animals are exposed to these spores. Pulmonary manifestation is the most common presentation in up to 75 percent of infected people [2]. Blastomycosis can also spread to skin, bones, genitourinary system, central nervous system. Among them, the most common sites of clinical disease are lung and skin [3]. The infection usually spreads through lymphatics and generate a pyogranulomatous reaction. Although initial infection results from inhalation of conidia into the lung in most of the cases, primary cutaneous blastomycosis rarely occurs after dog bites and accidental inoculation in the laboratory while performing autopsies [2]. Cutaneous inoculation blastomycosis is a rare illness, and there have been very few case reports of direct inoculation after trauma from dog bites and contaminated rocks without lung involvement [2],[4].
and might have contracted the blastomycosis spores during one of those hunting expeditions. In canine blastomycosis, lung involvement is seen in 65 to 85 % of cases and presents with cough, tachypnoea, cyanosis or respiratory distress [5],[6],[7],[8],[9],[10],[11],[12]. The patient and the canine did not show any signs of pulmonary infection. The dog started developing cutaneous lesions which later were also identified in our patient. The patient denied any trauma caused by the dog. We believe that dog had cutaneous infection blastomycosis which then spread to our patient from direct contact through minor scratches on his face and leg. The fact that the patient has not been exposed to endemic regions of blastomycosis makes it rather less likely that he might have developed the skin lesion from pulmonary spread. The pulmonary spread also could not be completely ruled out as sometimes the infections can be asymptomatic and might have gone unnoticed. As bronchoscopy is not routinely done in these patients, it is difficult to exclude pulmonary infection. Further studies have to be done to look into direct inoculation of blastomycosis. More studies could shed light into whether cutaneous blastomycosis patients should have contact isolation if they were found to be infected.
The case provides another interesting link in the relationship between dog, man, and direct inoculation blastomycosis.However, more studies are needed investigating direct inoculation of blastomycosis as it may be required to not just treating the patients but also the possible contacts as they might serve as the nidus of persistent / future infections.
CONCLUSION
Definite history of trauma might not be available, and there should be a high index of suspicion for cutaneous blastomycosis when considering a patient presenting with a non-healing ulcer from regions where prevalence of blastomycosis is high. Animal contacts should be enquired and if infected should be treated as they can remain as a nidus of further infections.
REFERENCES
1.
Bradsher RW Jr. The endemic mimic: Blastomycosis an illness often misdiagnosed. Trans Am Clin Climatol Assoc 2014;125:188–202.
[Pubmed]

2.
Kuzel AR, Lodhi MU, Syed IA, et al. Cutaneous, intranasal blastomycosis infection in two patients from Southern West Virginia: Diagnostic dilemma. Cureus 2018;10(1):e2095. [CrossRef]
[Pubmed]

3.
Smith JA, Kauffman CA. Blastomycosis. Proc Am Thorac Soc 2010;7(3):173–80. [CrossRef]
[Pubmed]

4.
Emer JJ, Spear JB. Primary cutaneous blastomycosis as a cause of acute respiratory distresssyndrom: Case report and literature review. J Clin Aesthet Dermatol 2009;2(3):22–30.
[Pubmed]

5.
Chapman SW, Dismukes WE, Proia LA, et al. Clinical practice guidelines for the management of blastomycosis: 2008 update by the infectious diseases society of America. Clin Infect Dis 2008;46(12):1801–12. [CrossRef]
[Pubmed]

6.
Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev 2010;23(2):367–81. [CrossRef]
[Pubmed]

7.
Gray NA, Baddour LM. Cutaneous inoculation blastomycosis. Clin Infect Dis 2002;34(10):E44–9. [CrossRef]
[Pubmed]

8.
Sarosi GA, Eckman MR, Davies SF, Laskey WK. Canine blastomycosis as a harbinger of human disease. Ann Intern Med 1979;91(5):733–5.
[Pubmed]

10.
Kerl ME. Update on canine and feline fungal diseases. Vet Clin North Am Small Anim Pract 2003;33(4):721–47. [CrossRef]
[Pubmed]

11.
Arceneaux KA, Taboada J, Hosgood G. Blastomycosis in dogs: 115 cases (1980–1995). J Am Vet Med Assoc 1998;213(5):658–64.
[Pubmed]

12.
Brömel C, Sykes JE. Epidemiology, diagnosis, and treatment of blastomycosis in dogs and cats. Clin Tech Small Anim Pract 2005;20(4):233–9. [CrossRef]
[Pubmed]

SUPPORTING INFORMATION
Author Contributions
Aparna Baburaj - Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Anuraj Sudhakaran - Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Divya Singh - Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Siddharth Chand - Substantial contributions to conception and design, Acquisition of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published
Guaranter of SubmissionThe corresponding author is the guarantor of submission.
Source of SupportNone
Consent StatementWritten informed consent was obtained from the patient for publication of this case report.
Data AvailabilityAll relevant data are within the paper and its Supporting Information files.
Conflict of InterestAuthors declare no conflict of interest.
Copyright© 2019 Aparna Baburaj et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information.