|
|
Editorial
| ||||||
| The problem of leprosy: Past, present and future | ||||||
| Mauro Rubini | ||||||
|
SABAP-RM-MET, Head of the Anthropological Service, Rome, Italy
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article] [Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Rubini M. The problem of leprosy: Past, present and future. Edorium J Infect Dis 2018;4:1–4. |
|
INTRODUCTION
| ||||||
|
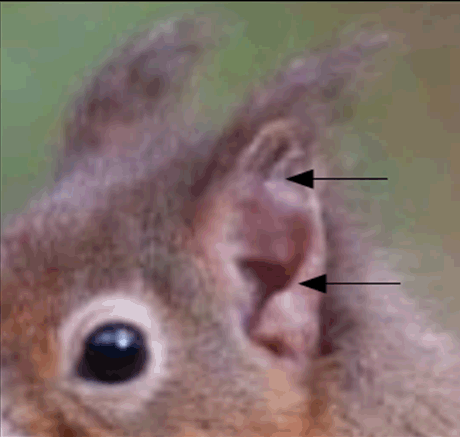
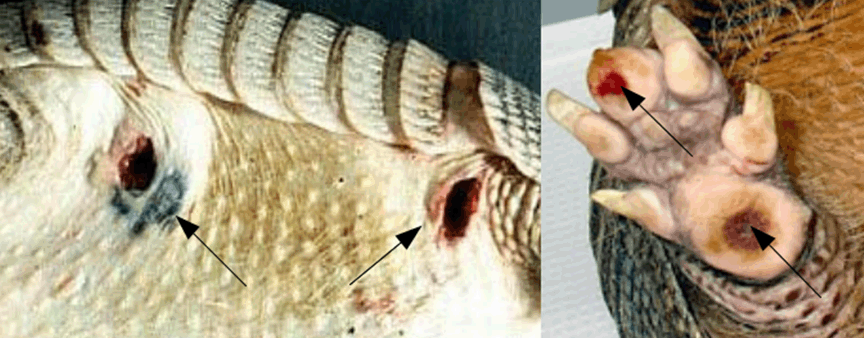
Leprosy is an infectious disease with low pathogenicity, caused by uncultivable obligate pathogens Mycobacterium leprae, that causes the classical disease, and the recently discovered Mycobacterium lepromatosis that can produced the same type of lesions [1]. It is not directly fatal. The disease can be contracted by inhalation of bacteria-laden droplets from the nose or by direct contact with leper sick. Recently, the M. lepromatosis was isolated in red squirrels (Figure 1) in England and British Island [2]. The meat of these squirrels was very appreciated during the middle age and according some scholars the zoonotic transmission was the cause of the great number of lepers in England during this period. Also in same southern states of the USA and Central America the Mycobacterium leprae is present in another animal: armadillo of Dasypus novemcinctus species (Figure 2). Also in this case the zoonotic transmission was suspected in same case [3]. Furthermore, during the 90 years Mycobacterium was isolated in some species of monkeys [4]. Mycobacterium was also isolated in the soil and water puddle nearby to human settlements where leprosy is present [5]. The early signs and symptoms of leprosy are subtle and with a slow clinical development. The incubation period may last over 20 years (in some rare cases 40 years). Nerves, skin and soft tissues are involved. Furthermore, M. leprae can also be detected in bone tissue [6]. In particular maxillo-facial region and hard palate are the more involved areas [6]. The sensory loss is the first symptoms [7][8]. When M. leprae is present in the nerve and soft tissue a series of typical skeletal changes can be observed (although in low percentage 5–7%), as mentioned above mainly in the rhino-maxillary area, and secondarily also in the bones of the hand and foot. Furthermore, non-specific changes can occur in the tibia and fibula [9]. Leprosy shows a spectrum of clinical manifestations related to the host immunological status. The extremes are multibacillary (or lepromatous) leprosy with a strong humoral but ineffective response associated with large numbers of M. leprae or lepromatosis, and paucibacillary (or tuberculoid) leprosy, with few visible M. leprae in tissues but a strong cell-mediated response [10][11]. Between the clinical extremes of multibacillary and paucibacillary leprosy, there are the borderline types [9]. In addition to these groups that manifest clinical leprosy, individuals may be infected with the Mycobacterium without developing the clinical signs of the disease. This subtle situation is called subclinical leprosy [7][8]. According to some authors [12][13] the incidence of subclinical leprosy is high today, as it likely was in the past [14]. Although multibacillary leprosy is the most invalidant and infectious form, in the past but also today where there are poor hygienic, living and diet conditions, and paucibacillary leprosy the less infectious, in a general consideration of the disease is important to evaluate also the subtle contagiousness of the other forms of leprosy clinically less manifest or invisible as the subclinical forms [13]. These last probably play an important role in the spread of the disease, especially in developing countries or where there is no capillarity of diagnostic centers (hospitals, laboratory of microbiological analysis). | ||||||
| Is there divergence in mortality trend of leprosy between pre-antibiotic and post-antibiotic era? | ||||||
|
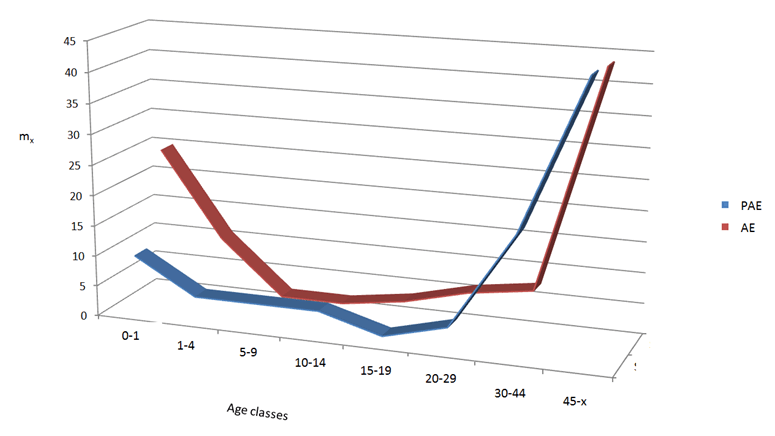
The mortality trend (curve) of a population that includes an infectious disease highlights not only the mortality rate caused by the disease but also the deaths from other causes. Leprosy is not a fatal disease. The lepers died for superinfections. Fatal superinfection can occur in short, medium or long times depends on the health general condition of the patient and above all, today, whether he is treated or not. The abridged life table was obtained with the TABRIMOR software [15]. The proposed model is defined credibility-adjusted [16]. An abridged life table is a matrix, showing changes in a standard set of life table functions (columns) across ages (rows). The conventional set of life table functions includes: dx - number of deaths within the elementary age interval, mx - death rate (x 100) for the elementary age interval, lx - probability of survival from birth to age x, qx - probability of death within elementary age interval, Lx - number of person-years lived within the elementary age interval, Tx - number of person-years lived after the age x, ex - life expectancy at age x. To create the mortality curves only the mx was used because they allowed a comparison with data from literature and/or presented on WHO reports. The age classes used were every five years for sub-adults, with the exception of the peri-neonate (0–1 and 2–4 years) age, while for adults we subdivided the sample in three age classes: young adult (20–29 years), adult (30–44 years) and mature adult (45–x years). This has allowed a more detailed analysis of mortality trend [15]. Some mortality curves of past and present populations with leprosy were built to compare eventual different trend in mortality. The antibiotic era for the leprosy can be started during the 40’ years with the discovery of the sulfons but only in 1982 when the WHO introduced the use of multidrug therapy with dapsone, rifampicina, clofazimine, prednisone and thalidomide which were obtained satisfactory clinical results. Even for some superinfections with the use of antibiotics, some results have been achieved, for example in tuberculosis to start from the 20th century. The comparison (Figure 3) between a population of 7th century with leprosy from central Italy [17] and a present day population with leprosy from northeastern Brazil (WHO, 2015) shows the trend of mortality in pre-antibiotic and post-antibiotic antibiotic era. The two curves are very similar with the only exception of the perinatal mortality that is greater in the past. As mentioned above the leprosy is not a fatal diseases and for this reason no produced mortality peak like other fatal infectious diseases for example plague or cholera. The distribution of health factor risks is similar to that of a normal population in both the samples, they are distributed along the life cycle. This result highlights that the leprosy problem is not only due to the absence of specific drugs in the past but also to other deficiencies today. Furthermore in recent times, cases of resistance to multidrug therapy due to mutations of Mycobacterium leprae have also been reported [18]. Is the leprosy a rural disease? Often this affirmation was validate by some scholars in comparison, for example, with the plague or tuberculosis. In particular, the latter is strongly associated with the phenomenon of urbanization. Probably the leprosy is a disease of the poverty. Today this problem is often associated with deficiencies in clinical facilities and infrastructures especially in developing countries. Meanwhile, it should be pointed out that perhaps the estimates of leprosy patients in the world are unreliable. Some countries underestimate the incidence of cases such as USA, Russia and China. It may seem paradoxical but this phenomenon is linked to the fact that countries that export images of well-being, comfort and good living standards, consider detrimental to show the other side of the coin. The presence of a disabling, disfiguring disease and above all an emblem of poverty probably it must be kept hidden in its integrity and shown only as a casual, marginal and sporadic occurrence. We know that leprosy is present in the territories of the south of the USA, as well as in the endless Chinese countryside where the lack or difficulty of reaching hospital centers is often impossible. Obviously, if this happens among the poor agricultural populations of south-eastern Brazil or in Africa or in the extra-urban areas of India (where otherwise there is a big consideration of the problem), it is all normal but in countries where the image of the well-being it serves to stimulate productivity and economy, perhaps leprosy is a disease to be hidden in the past as it is today. Keywords: Antibiotic-era, Leprosy, M. leprae, Mortality trend | ||||||
| ||||||
| ||||||
| ||||||
|
REFERENCES
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text] |
|
Author Contributions
Mauro Rubini – Substantial contributions to conception and design, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of Submission
The corresponding author is the guarantor of submission. |
|
Source of Support
None |
|
Conflict of Interest
Author declares no conflict of interest. |
|
Copyright
© 2018 Mauro Rubini. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|